Convalescent plasma treatment of hospitalized patients with COVID-19 has been subject to Emergency Use Authorization (EUA) since August of 2020. Subsequent scientific studies have indicated the need to modify this EUA by limiting use to immunocompromised patients. A new HCPCS code C9507 was created to describe this service (C9507 Long Descriptor: Fresh frozen plasma, high titer COVID-19 convalescent, frozen within 8 hours of collection, each unit).
On February 10, 2022, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the emergency use of COVID-19 convalescent plasma with high titers of anti-SARS-CoV2 antibodies for the treatment of COVID-19 patients with immunosuppressive disease or receiving immunosuppressive treatment in either the inpatient or outpatient setting.
Effective for dates of service on or after December 28, 2021, procedure code C9057 (Administration of high titer COVID-19 convalescent fresh frozen plasma in the outpatient) is a benefit. Procedure code C9507 is applicable for the diagnosis code U071. Clients of all ages, including pregnant women and children who meet EUA criteria, may be eligible for procedure code C9507.
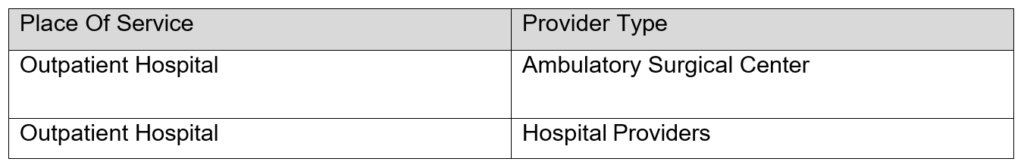
Procedure code C9507 can be administered by the following providers and places of service:
HHSC will cover convalescent plasma as a non-risk payment.
Note: prior authorization will not be required for procedure code C9507 in fee-for- service.
Convalescent Plasma EUA Letter of Authorization 12282021 (fda.gov)
MM12666 – April 2022 Update of the Hospital Outpatient Prospective Payment System (OPPS) (cms.gov)
For additional information, please contact the Provider Relations Division by phone at
(210) 358-6294 or by email at ProviderRelations@CFHP.com.
